The Food and Drug Administration sent a flurry of warning letters this week over bogus homeopathic products falsely claiming to be antiviral cures—products mostly marketed to children.
Amazon, Walmart, and the homeopathic company behind Naturasil products were among those receiving warnings for allegedly selling unapproved drugs in violation of federal regulations. The products are “especially concerning from a public health perspective because they are marketed for use in children,” the FDA wrote in its letter to Amazon.
The regulator identified four products on Amazon that were in violation. All of the products claimed to treat molluscum contagiosum and three were identified as homeopathic products. One of the products was Naturasil’s “Molluscum Treatment Kit,” which was also the focus of the letter to Walmart.
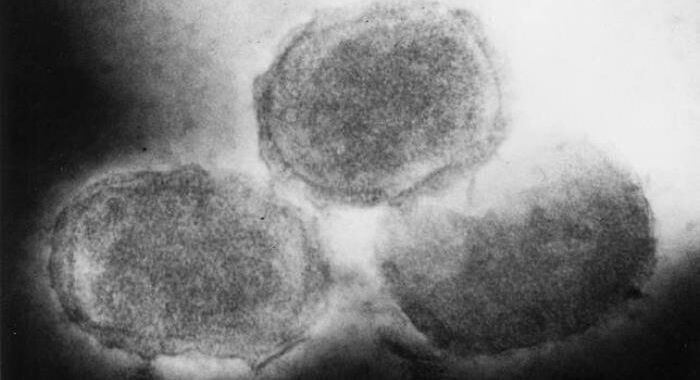
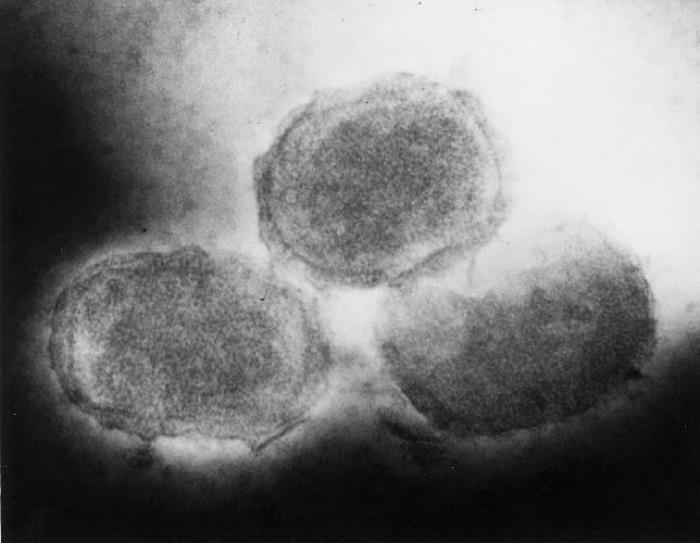
Molluscum contagiosum is a common skin infection caused by a poxvirus called molluscum contagiosum virus. Although anyone can be infected with the virus, it most often spreads in children. It infects the top layer of skin, causing lesions that tend to have dimpled centers. The rash is generally considered mild, though it can be red, itchy, and/or sore. The infection and lesions usually clear on their own, but it often takes six months to a year, and, in some cases, can take as long as four years.
Harms of homeopathy
Although molluscum contagiosum isn’t considered a particularly dangerous infection on its own, the FDA says it cannot be self-diagnosed and treated. Molluscum contagiosum can look similar to skin cancers, fungal infections, and—based on its location—sexually transmitted genital warts. “A healthcare professional is needed to rule out the possibility of a more serious condition,” the FDA wrote. Without that, the FDA worries consumers are “forgoing or delaying proper diagnosis and treatment of a potentially serious, undiagnosed health condition.”
This is a common concern for homeopathic products, which are based on pseudoscience and ineffective beyond the placebo effect. Homeopathy dates back to the late 18th century and relies on two unscientific principles: first, if a substance causes a specific symptom, it can treat conditions and diseases that include that symptom (“like cures like”); and, second, the more diluted the substance is, the more potent it is at treating medical conditions (the “law of infinitesimals”).
Often, homeopathic medicines are so diluted they contain nothing more than water and/or filler ingredients. Thus, safety is often not a concern—unless the products are improperly diluted or contaminated. In 2016, the FDA warned parents against using homeopathic teeth products after they were linked to 10 infant deaths and 400 cases of serious adverse events, including babies having seizures, losing consciousness, and turning blue. The symptoms and outcomes were consistent with poisoning with belladonna (aka deadly nightshade), the active ingredient in the teething products that was supposed to be diluted out. But, the FDA later found inconsistent amounts of belladonna in the products, some of which “far exceeded” what should have been present.
In the case of the molluscum contagiosum homeopathic products, the active, diluted ingredients included extracts from Thuja Occidentalis, an evergreen coniferous tree sometimes called a northern or eastern white cedar. The plant contains the poison thujune, a neurotoxin that causes convulsions. It’s most famous for being a toxic agent in absinthe. But homeopaths believe Thuja Occidentalis tinctures treat warts, among other things.
Regulatory crackdown
For decades, homeopathic products were allowed to be sold in the US without the FDA’s pre-market approval of safety and efficacy, though they are still regulated by the FDA. And, unlike dietary supplements, homeopathic products could make specific health claims about treating conditions, as long as the claims related to self-limiting conditions, like colds. But, in recent years, amid a booming alternative medicine industry, the FDA cracked down. Last year, it finalized new guidance using a risk-based approach to regulating unapproved homeopathic products. That is, it now flexes its regulatory discretion on any homeopathic products it considers high risk of causing harm.
In the warning letters, the FDA noted that it considers the molluscum contagiosum homeopathic products as those “potentially posing higher risks to public health,” and as such, would be prioritized for enforcement and regulatory actions. However, the FDA said it actually does not consider these products homeopathic ones because the”inactive” ingredients listed on the products’ labels are actually “active” in its eyes, putting the products squarely in the category of regulated drug products. For the Naturalis product, the inactive ingredients are listed as “Cedar Leaf Oil, Melaleuca Alternifolia Leaf Oil, Pale Pressed Ricinus Communis Seed Oil.”
The FDA gave Amazon, Walmart, and Naturalis 15 business days to respond to the letters. Naturalis did not respond to questions from Ars. (The FDA also warned the company over selling a homeopathic treatment for shingles.) Amazon did not immediately respond to a comment request either. However, searches for the products identified by the FDA indicated they were all now unavailable on Amazon and Walmart’s websites.