Waipapa Taumata Rau/University of Auckland
There’s rarely time to write about every cool science-y story that comes our way. So this year, we’re once again running a special Twelve Days of Christmas series of posts, highlighting one science story that fell through the cracks in 2022, each day from December 25 through January 5. Today: Scientists in New Zealand and Australia created tiny metallic snowflakes.
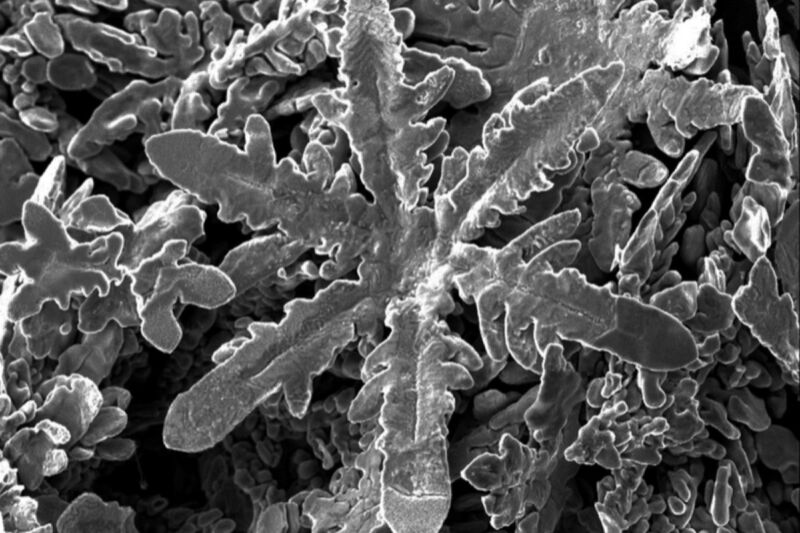
Scientists in New Zealand and Australia were conducting atomic-scale experiments with various metals dissolved in liquid solvent of gallium when they noticed something unusual: different types of metal self-assembled into different shapes of crystals—with zinc creating tiny metallic snowflakes. They described their results in a paper published earlier this month in the journal Science.
“In contrast to top-down approaches to forming nanostructure—by cutting away material—this bottom-up approaches relies on atoms self-assembling,” said co-author Nicola Gaston of University of Auckland. “This is how nature makes nanoparticles, and is both less wasteful and much more precise than top-down methods. There’s also something very cool in creating a metallic snowflake!”
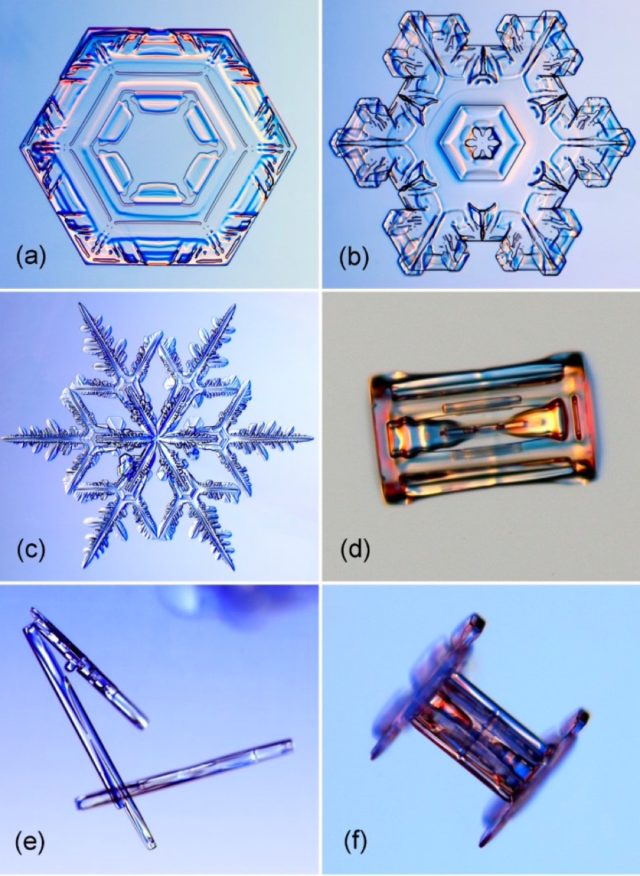
Snowflakes are the best known example of crystal growth, at least among the general populace. It’s long been known that under certain conditions, water vapor can condense directly into tiny ice crystals, usually forming the shape of a hexagonal prism (two hexagonal “basal” faces and six rectangular “prism” faces). But that crystal also attracts more cooled water drops in the air. Branchings sprout out from the single crystals’ corners to form snowflakes of increasingly complex shapes.
The shapes of snowflakes and snow crystals have long fascinated scientists, like Johannes Kepler, who took some time away from his star-gazing in 1611 to publish a short paper entitled “On the Six-Cornered Snowflake.” He was intrigued by the fact that snow crystals always seem to exhibit a six-fold symmetry. Some 20 years later, Rene Descartes waxed poetical after observing much rarer 12-sided snowflakes, “so perfectly formed in hexagons and of which the six sides were so straight, and the six angles so equal, that it is impossible for men to make anything so exact.” He pondered how such a perfectly symmetrical shape might have been created, and eventually arrived at a reasonably accurate description of the water cycle, adding that “they were obliged to arrange themselves in such a way that each was surrounded by six others in the same plane, following the ordinary order of nature.”
Robert Hooke’s Micrographia, published in 1665, contained a few sketches of snowflakes he observed under his microscope. But nobody performed a truly systematic study of snow crystals until the 1950s, when a Japanese nuclear physicist named Ukichiro Nakaya identified and cataloged all the major types of snow crystals. Nakaya was the first person to grow artificial snow crystals in the laboratory. In 1954 he published a book on his findings: Snow Crystals: Natural and Artificial.
Watch a snowflake “grow” into an intricate crystal structure. Credit: Kenneth Libbrecht
Thanks to Nakaya’s pioneering work, we know that certain atmospheric conditions, like temperature and humidity, can influence a snowflake’s shape. Star-like shapes form at -2 degrees Celsius and -15 degrees Celsius, while columns form at -5 degrees Celsius and again at around -30 degrees Celsius. And the higher the humidity, the more complex the shape. If the humidity is especially high, they can even form into long needles or large thin plates.
Kenneth Libbrecht, a physicist at Caltech, has been studying and photographing the formation of snowflakes for more than two decades. And like Nakaya, he also creates his own snowflakes in the lab, carefully using a small paintbrush to transfer the delicate structures to a glass slide, taking pictures with a digital camera mounted on a high-resolution microscope. He has documented the many kinds of snow crystals over the all those years, culminating in a 540-page monograph that has been called a tour de force of snowflake physics.
Most recently, in 2019, Libbrecht developed what he termed a “semi-empirical” model of the atomic processes at work to explain why there are two primary types of snowflakes: the iconic flat star, with either six or 12 points, and a column, sometimes sandwiched by flat caps and sometimes resembling a bolt from a hardware store. Libbrecht wanted to explore precisely what changes with the shifts in temperature. His model incorporates a phenomenon called surface-energy-driven molecular diffusion. Per Quanta:
A thin, flat crystal (either plate-like or starlike) forms when the edges rope in material more quickly than the crystal’s two faces. The burgeoning crystal will spread outward. However, when its faces grow faster than its edges, the crystal grows taller, forming a needle, hollow column or rod. According to Libbrecht’s model, water vapor first settles on the corners of the crystal, then diffuses over the surface either to the crystal’s edge or to its faces, causing the crystal to grow outward or upward, respectively. Which of these processes wins as various surface effects and instabilities interact depends mostly on temperature.
Kenneth Libbrecht
With this latest work, Gaston and her colleagues extended the analogy of ice snowflakes to metals. They dissolved samples of nickel, copper, zinc, tin, platinum, bismuth, silver, and aluminum in gallium, which turns liquid at just above room temperature, making it an excellent liquid solvent for the experiments. Once everything cooled, the metallic crystals formed but the gallium remained liquid. They were able to extract the metallic crystals by reducing the surface tension of the gallium solvent—achieved via a combination of electrocapillary modulation and vacuum filtration—and carefully documented the different morphologies of each.
Next they conducted simulations of the molecular dynamics to determine why different metals produced differently shaped crystals: cubes, rods, hexagonal plates, and in the case of zinc, a snowflake structure. They found that it all comes down to the interactions between the atomic structure of the metals and the liquid gallium. “What we are learning is that the structure of the liquid gallium is very important,” said Gaston. “That’s novel because we usually think of liquids as lacking structure or being only randomly structured.”
DOI: Science, 2022. 10.1126/science.abm2731 (About DOIs).
Listing image by Waipapa Taumata Rau/University of Auckland