A Pfizer vaccine designed to protect newborns and infants from severe RSV illness won a recommendation from the Centers for Disease Control and Prevention Friday—but only for seasonal use.
The vaccine is Pfizer’s bivalent RSVpreF vaccine, called Abrysvo, and is administered to pregnant people late in gestation, between 32 and 36 weeks.
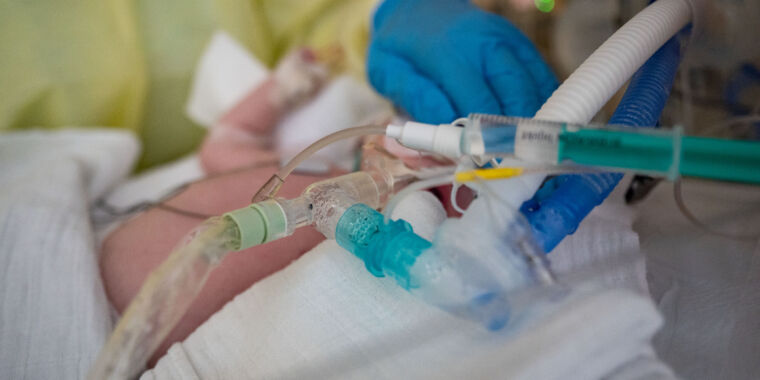
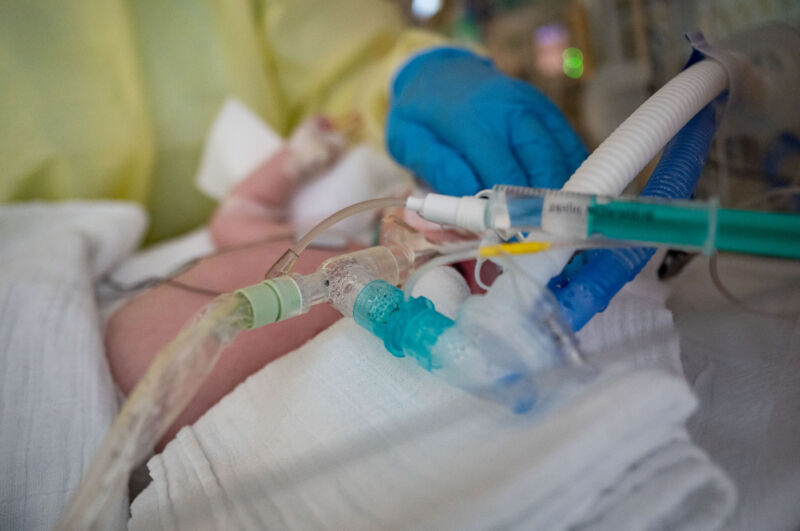
RSV, or respiratory syncytial (sin-SISH-uhl) virus, is the leading cause of hospitalization for infants in the US. Each year, 1.5 million children seek out-patient care for RSV, with 58,000 to 80,000 ending up in the hospital and 100 to 300 tragically dying from the infection.
The CDC’s Advisory Committee on Immunization Practices voted 11 to 1 on Friday in favor of the limited recommendation for the vaccine, which in a clinical trial appeared 91 percent effective at preventing severe RSV in the first three months of a baby’s life and 76.5 percent effective against severe disease in the first six months. It demonstrated 57 percent efficacy in preventing hospitalization in the first six months.
The vaccine did appear to increase the pre-term birth rate compared with placebo, but the increase was not statistically significant.
Ultimately, the committee only recommended the vaccine to be used seasonally—between September and January to protect babies born between October and March, when RSV transmission typically peaks. (There is an exception for pregnant people who live in an area of the US where RSV circulates year-round, such as Hawaii and Gaum.)
For pregnant people whose babies are due between February and August, the vaccine is not recommended. Instead, those babies will have the option of a monoclonal antibody immunization by Sanofi, called nirsevimab (Beyfortus), available to protect against RSV in the run-up to the seasonal transmission. The antibody has been shown to be about 80 percent effective at preventing severe RSV over five months.
The one dissenting vote on the CDC’s committee was Helen Keipp Talbot, a medical professor at Vanderbilt University, who questioned the complexity of the recommendation and the need for another option, given the availability of the antibody. But other members highlighted the benefits of having two options available.
Shortly after the advisory committee’s vote, CDC Director Mandy Cohen endorsed the recommendation.
“This is another new tool we can use this fall and winter to help protect lives,” Cohen said in a statement. “I encourage parents to talk to their doctors about how to protect their little ones against serious RSV illness, using either a vaccine given during pregnancy, or an RSV immunization given to your baby after birth.”
Both options come at steep prices. Pfizer plans to charge $295 for its shot, and Sanofi sells the monoclonal immunization for $495.