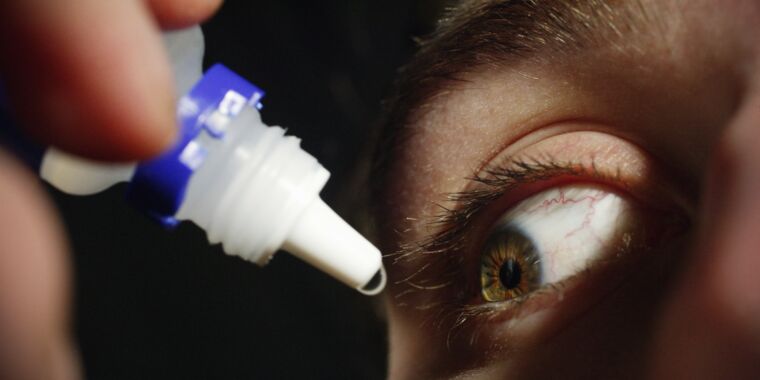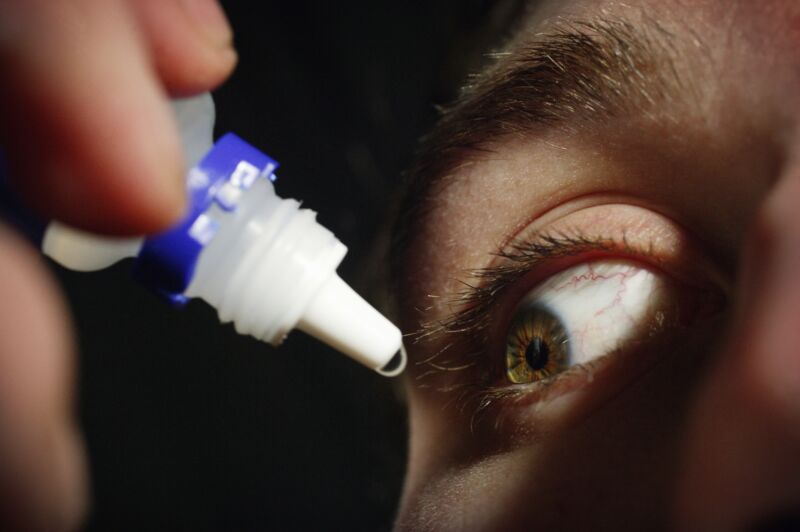
Two more people have died and more details of horrifying eye infections are emerging in a nationwide outbreak linked to recalled eye drops from EzriCare and Delsam.
The death toll now stands at three, according to an outbreak update this week from the Centers for Disease Control and Prevention. A total of 68 people in 16 states have been infected with a rare, extensively drug-resistant Pseudomonas aeruginosa strain linked to the eye drops. In addition to the deaths, eight people have reported vision loss and four have had their eyeballs surgically removed (enucleation).
In a case report published this week in JAMA Ophthalmology, eye doctors at the Bascom Palmer Eye Institute, part of the University of Miami Health System, reported details of one case linked to the outbreak—a case in a 72-year-old man who has an ongoing infection in his right eye with vision loss, despite weeks of treatment with multiple antibiotics. When the man first sought treatment he reported pain in his right eye, which only had the ability to detect motion at the point, while his left eye had 20/20 vision. Doctors noted that the white of his right eye was entirely red and white blood cells had visibly pooled on his cornea and in the front inner chamber of his eye.
The man’s eye tested positive for a P. aeruginosa strain resistant to multiple antibiotics—as did the bottle of EzriCare artificial tear eye drops he had been using. After further testing, doctors switched the man’s treatment plan to using hourly doses of antibiotics to which the bacterial strain was least resistant. At a one-month follow-up visit, the redness and eye infiltrates had improved in the man’s eye. But to date, the infection has persisted, the doctors reported, as has his vision loss. (Graphic images of his right eye at the initial presentation and one-month follow-up can be found here.)
Growing outbreak
The CDC identified the outbreak strain as VIM-GES-CRPA, which stands for a carbapenem-resistant P. aeruginosa (CRPA) with Verona integron-mediated metallo-β-lactamase (VIM) and Guiana extended-spectrum-β-lactamase (GES). This is an extensively drug-resistant strain that, before the outbreak, had never been seen in the US before. CDC officials fear the outbreak will lead to these types of infections becoming more common, as the bacteria can asymptomatically colonize in people, spread to others, and share their resistance genes.
Authorities believe that the outbreak strain was brought into the country in the contaminated eye drops, which were manufactured by Global Pharma, a Chennai, India-based manufacturer. The Food and Drug Administration reports that it has had a slew of manufacturing violations. The eye drops were imported into the country by Aru Pharma Inc. and then branded and sold by EzriCare and Delsam Pharma. The products were available nationwide via Amazon, Walmart, eBay, and other retailers.
ABC News on Thursday reported another case treated by doctors at the Bascom Palmer Eye Institute, in which a 68-year-old Miami woman lost an eye after using EzriCare drops. The woman, Clara Oliva, developed an infection in her right eye last August and went to the Institute for emergency care due to intense pain that she described as feeling like shards of glass in her eye. Doctors discovered the pain was due to a P. aeruginosa infection, but did not immediately link it to the eye drops. The doctors tried to surgically repair the eye but found extensive, irreparable damage and worried that the drug-resistant infection would spread. On September 1, they removed her infected eye entirely. Oliva, left legally blind by the enucleation and poor vision in her remaining eye, continued using the EzriCare eye drops until January when the CDC released the first advisory about the outbreak. She is now suing EzriCare, Global Pharma, the medical center that prescribed her the eye drops, and her insurer.
Oliva isn’t the only one filing lawsuits. Last month, Jory Lange, a Houston-based attorney with expertise in food safety, filed two lawsuits on behalf of women affected by the outbreak.
“I think this outbreak is, unfortunately, likely to continue to grow,” Lange told Ars. For one thing, people continue to be diagnosed, he said. But, the CDC has also advised clinicians to look at infections from early last year. As of now, the identified cases in the outbreak span from May 2022 to February 2023, but the CDC is advising clinicians to report any drug-resistant P. aeruginosa cases as far back as January 2022. “We’ve talked to some people who were infected in that early time frame, so we think their cases will end up being added,” Lange said.